
VHP Female Model v5.0
The VHP Female Model v5.0 is our most advanced, anatomically accurate, and numerically efficient computational human model. Qualified as a Medical Device Development Tool (MDDT) by the FDA in 2023, it is essential in characterizing the response of the human body to a variety of electromagnetic, acoustic, and other external stimuli.


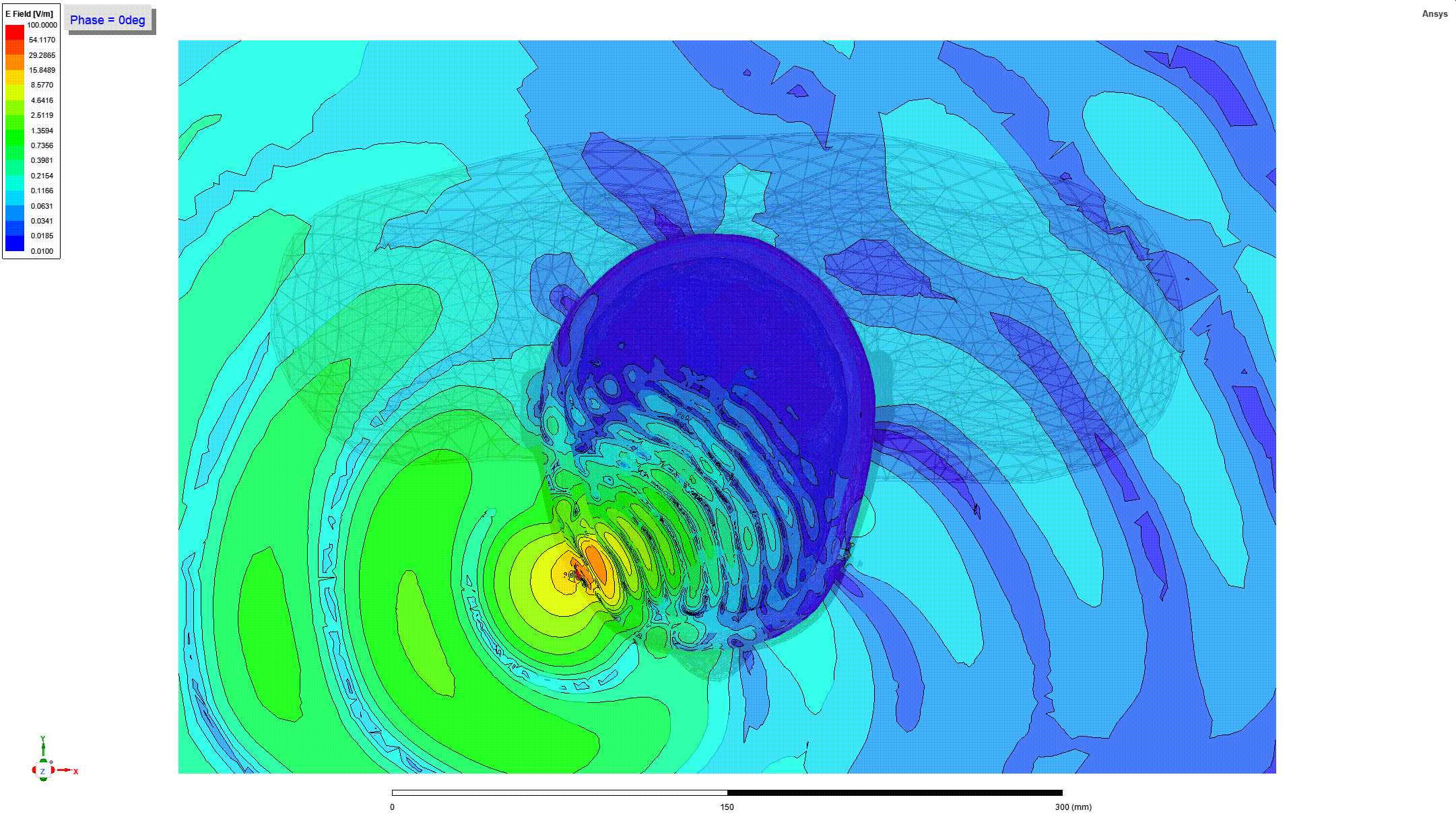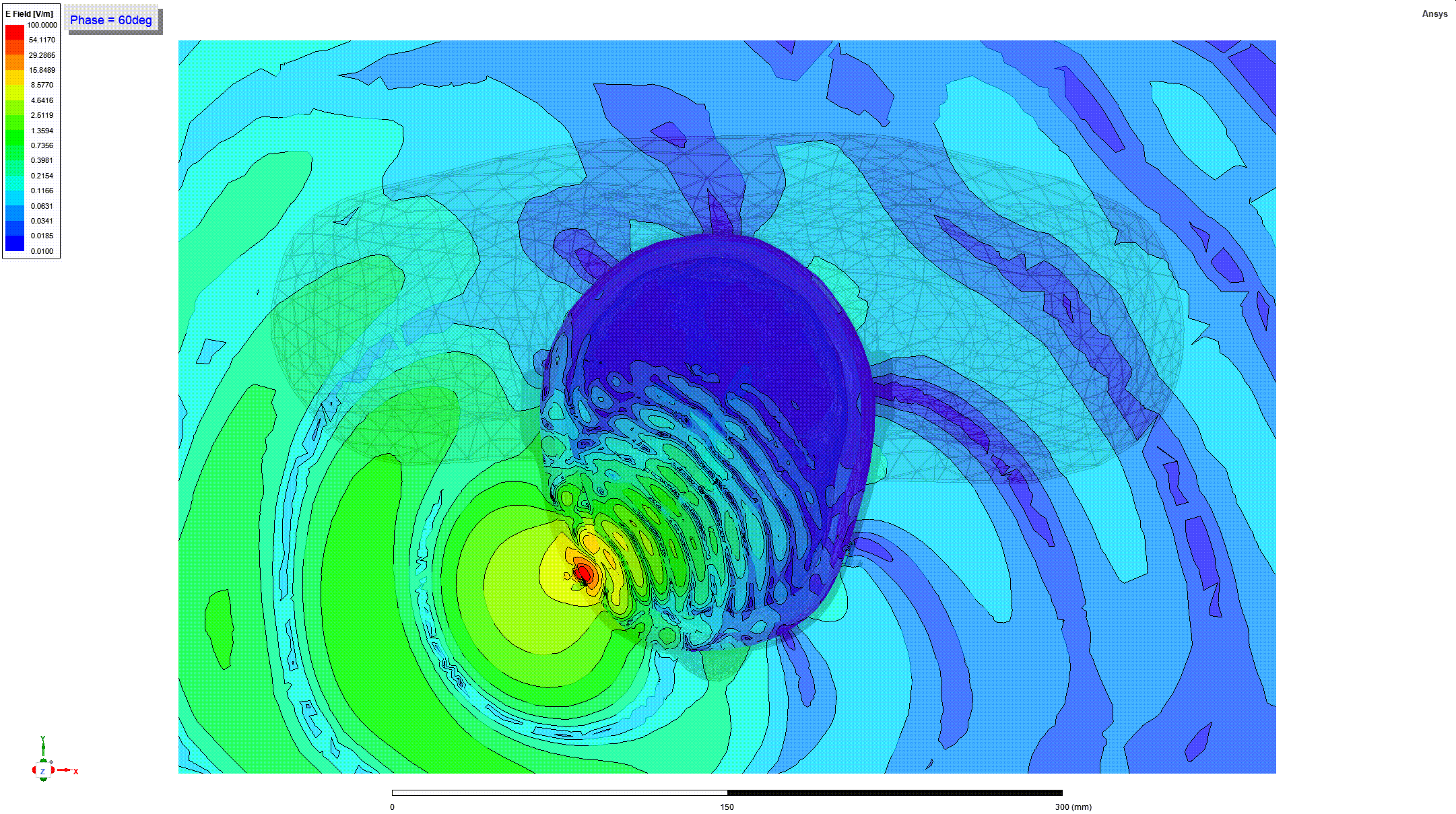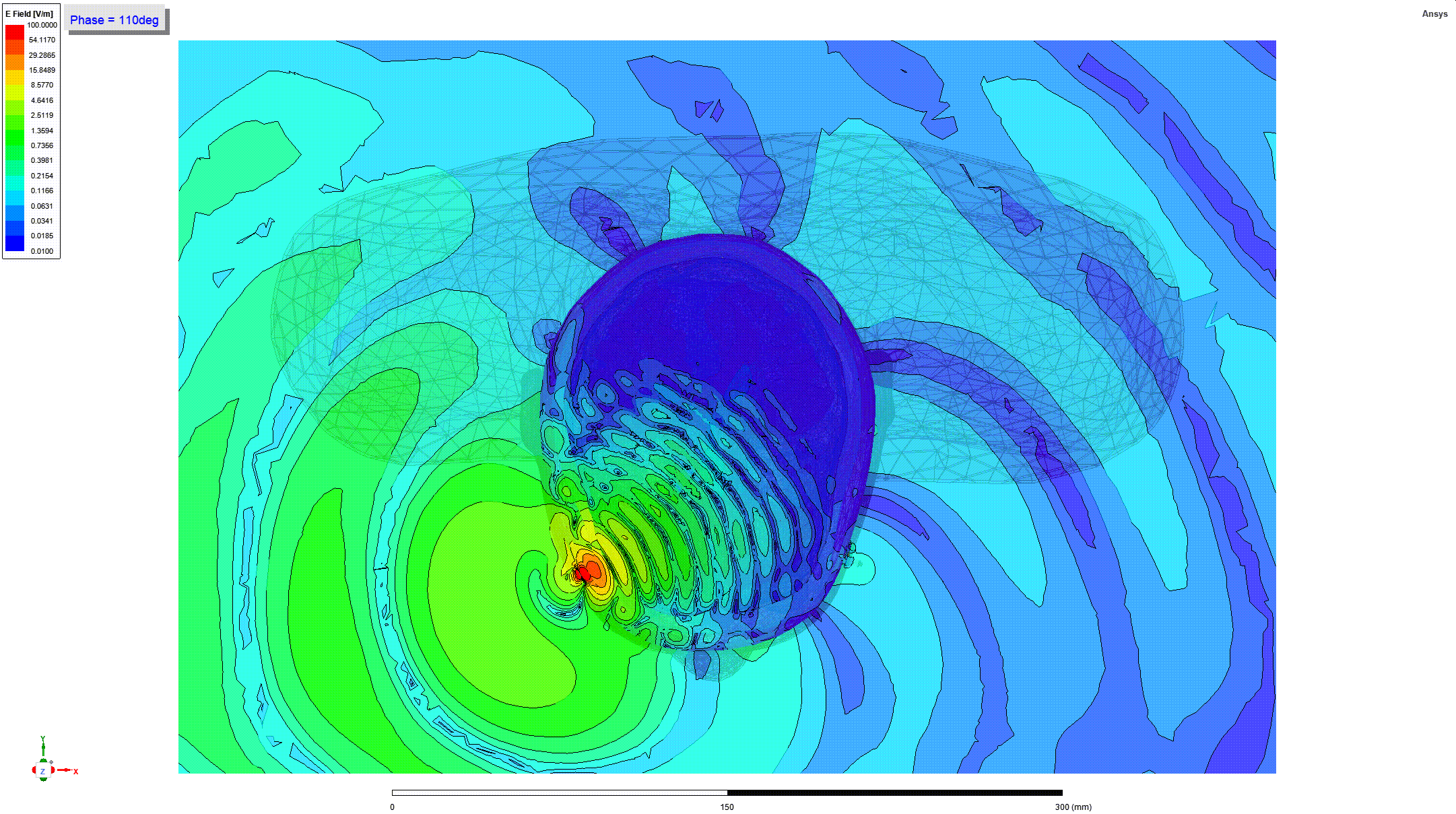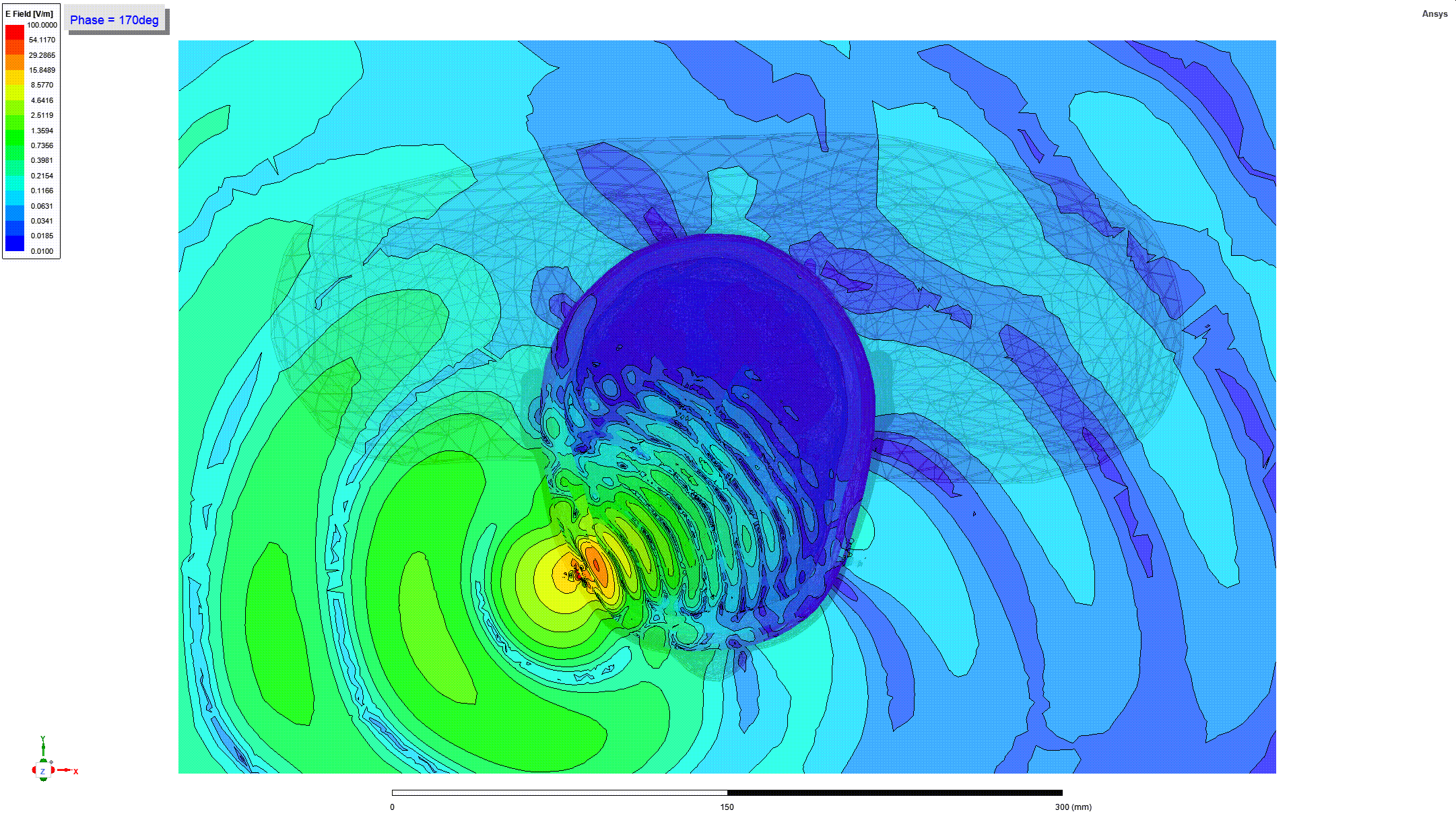
Details and Accuracy
The VHP Female Model v5.0 is based exclusively on medical data collected as part of the US Library of Medicine’s Visible Human Project - a collection of extremely detailed and anatomically accurate data obtained from a female cadaver. The data includes magnetic resonance imagery (MRI), computed tomography (CT) imagery, and high-definition photographs of cross-sectional cryo-sections.
The VHP Female v5.0 completed a thorough validation process where the mesh surface cross-sections overlay the original cryo-section images in every transverse plane (every 10 mm in the head, and every 25 mm in the body).
-
Over 250 Individual Parts
-
Approximately 50 Individual Muscles
-
Enhanced Spinal Cord with Separate White and Grey Matter
-
Pulmonary Artery Structure
-
High Definition Intra-cranial Segmentation
-
Highly Detailed Ear Canal with Inner Ear
-
Radial, Peripheral, Sciatic, and Median Nerves
-
3 Body Shells - Skin, Fat, and Average


FDA Qualified MDDT
The VHP Female Model v5.0 is a qualified Medical Device Development Tool (MDDT) categorized as a non-clinical assessment model (NAM) by The Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA).
The model can predict heating of tissues around metallic orthopedic implants when these implants are subjected to radio frequency (RF) electromagnetic fields generated by magnetic resonance imaging (MRI) coils (e.g. when a person undergoes an MRI scan).
These predictions can be used to generate parameters including the RF power deposition and induced temperature rise near passive metallic femoral intramedullary nails and screws (21 CFR 888.3020, product code HSB) as a function of implant geometry, location, material, and multiple scan times and cooling times.
Click here for the MDDT Summary.


Cross-Platform Models Meet Your Computational Needs
NEVA EM human models can run in any software package, either finite element, finite difference, or boundary element based. Current partners include: Ansys (HFSS), Dassault Systèmes (CST Studio Suite), and Cadence (Clarity 3D Solver).
Availability
The VHP Female Model v5.0 is available for commercial use. Please click the button below to license the VHP Female v5.0 model.
